By: Natalie A. Haddad, Diana J. Schreier, Jennifer E. Fugate, Ognjen Gajic, Sara E. Hocker, Calvin J. Ice, Sarah B. Leung, Kristin C. Mara, Alejandro A. Rabinstein, Andrew D. Rule & Erin F. Barreto
Published: 08 February 2022
Background
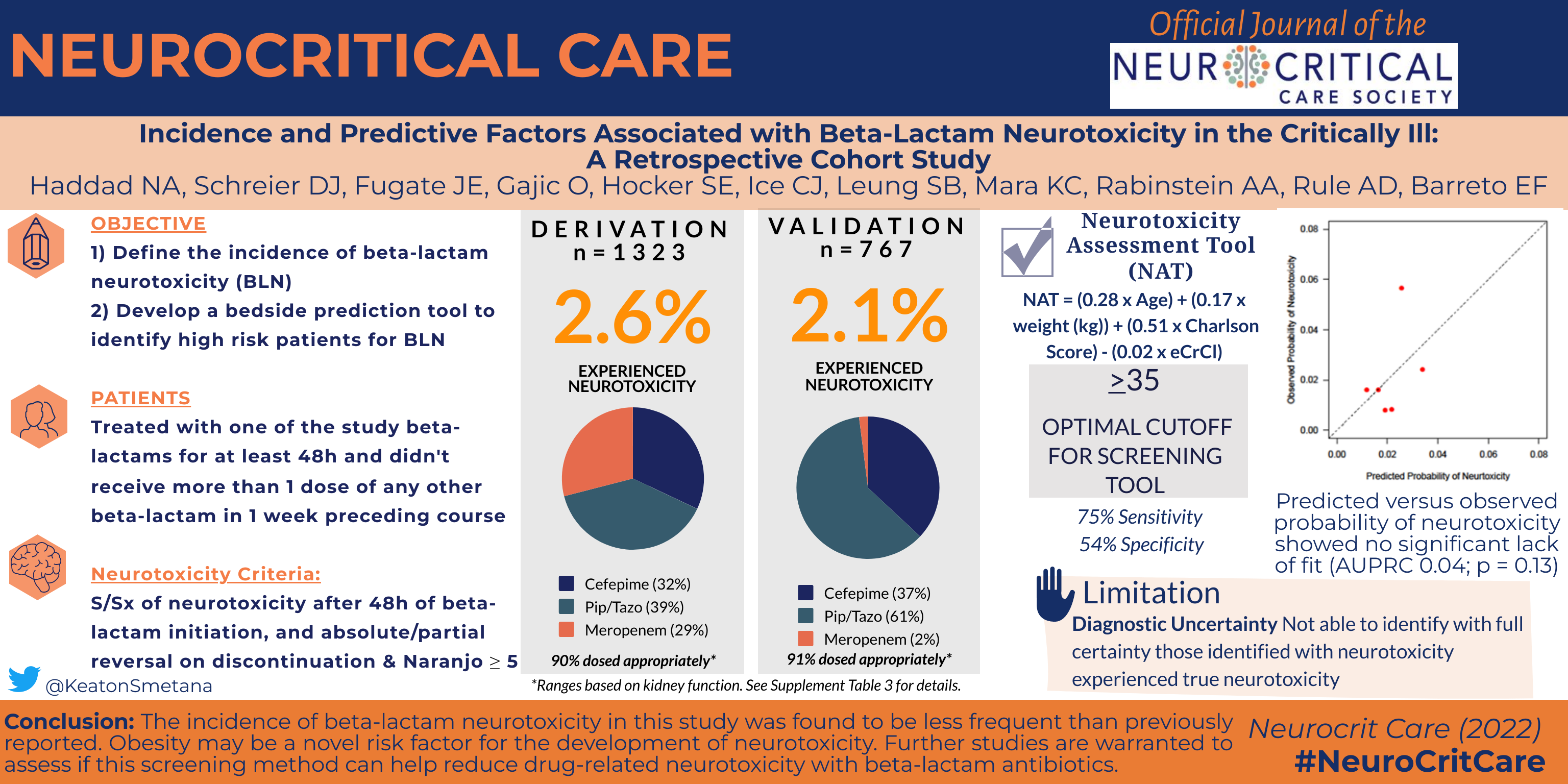
Beta-lactam neurotoxicity is a relatively uncommon yet clinically significant adverse effect in critically ill patients. This study sought to define the incidence of neurotoxicity, derive a prediction model for beta-lactam neurotoxicity, and then validate the model in an independent cohort of critically ill adults.
Methods
This retrospective cohort study evaluated critically ill patients treated with ≥ 48 h of cefepime, piperacillin/tazobactam, or meropenem. Two separate cohorts were created: a derivation cohort and a validation cohort. Patients were screened for beta-lactam neurotoxicity by using search terms and diagnosis codes, followed by clinical adjudication using a standardized adverse event scoring tool. Multivariable regression models and least absolute shrinkage and selection operator were used to identify surrogates for neurotoxicity and develop a multivariable prediction model.
Read the full article.